THE SOLUTION
WHY WILL 2P Revolutionize treatment?
Effectively penetrates the Blood Brain Barrier (BBB)
Kills multi-drug resistant HGG stem cells, DIPG and glioblastoma (GBM) cell lines at nanomolar concentrations
Synergistic with Temozolomide and Radiation
2P-Im nontoxic to normal human brain astrocytes
Inhibits Inflammasome activation Orally bioavailable; potential for orphan disease designation
Understanding the science
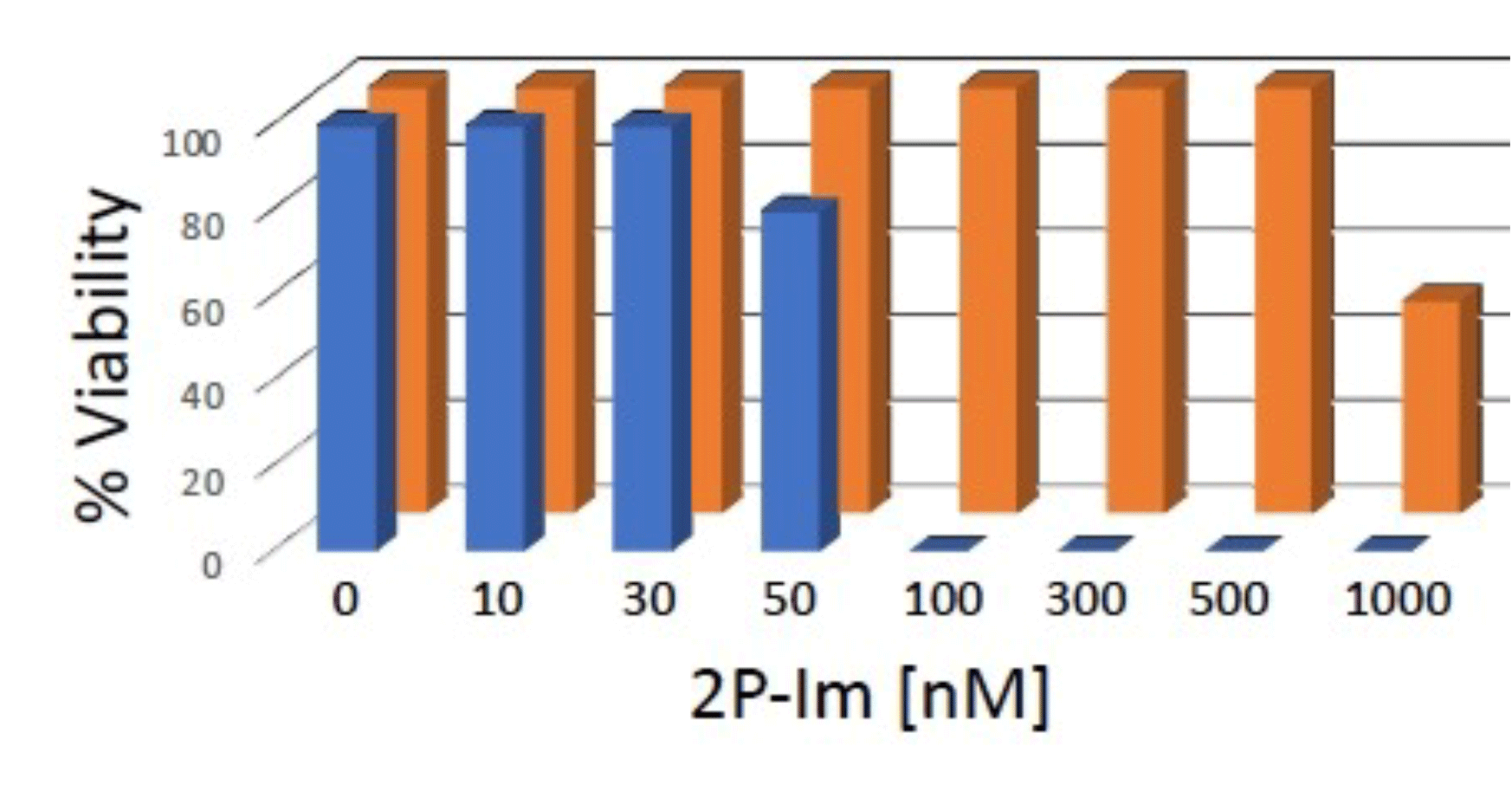
Induction of GBM Cell Death by 2P-Im
Primary cultures of human glioblastoma cells (blue) and of normal human astrocytes (orange) were exposed to concentrations of 2P-Im, from 10 to 1000 nanomolar. Complete GBM cell death was observed at 24 hours at 100nM, while astrocytes remained 100% viable at 500nM.
THE PROBLEM
Macrophages are the key to understanding the role of the tumor microenvironment. Macrophages promote the growth of most cancers and when activated drive the development of resistance to radiation, immunotherapy and chemotherapy.
2P-Im
Triterpenoid Therapeutics has developed 2P-Im, the most potent regulator of macrophage function that has ever been tested. 2P-Im and its related derivatives have the demonstrated capacity to complement and dramatically enhance the efficacy of currently approved therapies.
Data: 2P-Im is Active in High Grade Glioma (HGG)
2P-Im induces cell death in primary cultures of human GBM at Nanomolar concentrations.
2P-Im exhibits no toxicity in primary cultures of normal human astrocytes.
TMZ requires Micromolar concentrations to inhibit GBM
2P-Im sensitizes GBM stem cells to radiation
Our Answer
TTX’s lead compound, 2P-Im, is an anti-inflammatory agent and inhibitor of oxidative stress that targets the GBM tumor microenvironment. 2P-Im penetrates the blood-brain barrier and effectively kills GBM tumor cells. It is non-toxic to Astrocytes and non-tumor cells. 2P-Im inhibits inflammasome activity and blocks essential mitochondrial Stress Response Proteins (SRPs) that fuel GBM tumors. It targets tumor-induced immune suppression and helps overcome resistance. Its ability to synergize with the current GBM standard of care provides an opportunity for “fast track” FDA approval. 2P-Im is administered orally and has strong bioavailability.