LEAD Program
our lead compounds
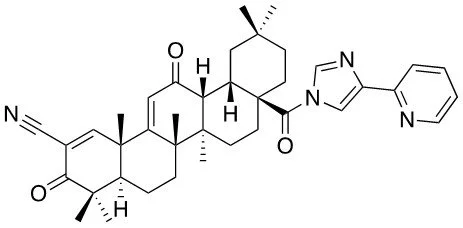
TTX-01 (CDDO 2P-IM)
TTX-01 is a fourth-generation synthetic triterpenoid designed as an oral, brain-penetrant therapy that complements existing standard of care.
Unprecedented Potency
Preclinical studies show TTX-01 is the most powerful triterpenoid developed to date with data demonstrating that it is 1000X more potent than earlier compounds and superior to leading NLRP3 inhibitors like MCC950.
Proven Potential in Brain Disease
In neurodegenerative disease models, data demonstrate that TTX-01 crosses the blood-brain barrier and reduces Alzheimer’s pathology including amyloid plaques, while protecting neurons from inflammatory damage.
In brain cancer models, data demonstrate that TTX-01 overcomes resistance to standard chemotherapy (TMZ), enhances radiation effectiveness, and significantly delays tumor progression in glioblastoma.
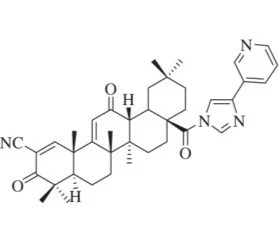
TTX-02 (CDDO 3P-Im)
In preclinical discovery models TTX-02 has demonstrated strong bioavailability and a capacity to disrupt NLRP3 Inflammasome assembly and modulate the pathways of the ISR at nanomolar concentrations. Currently in optimization, TTX-02 is intended for deployment in neurodegenerative applications.
Pipeline Assets
TTX pipeline features sixteen patent pending fluorinated analogs of its lead compounds. These next-generation compounds are orally bioavailable, and aim to further enhance brain penetration, increase selectivity, and maintain favorable safety profile.